Salusmo
ProSalusmoPro is a medical device under development, designed for the early detection of stroke by analyzing movement patterns.

SalusmoPro is an innovative medical technology under development, aiming to enable early detection of stroke through wearable motion sensors (Apple Watch, dedicated motion sensors) and advanced AI analysis of movement data.
The system utilizes machine learning algorithms trained to recognize movement pattern changes caused by neurological dysfunction.

Every second counts
Stroke as a Global Health Crisis
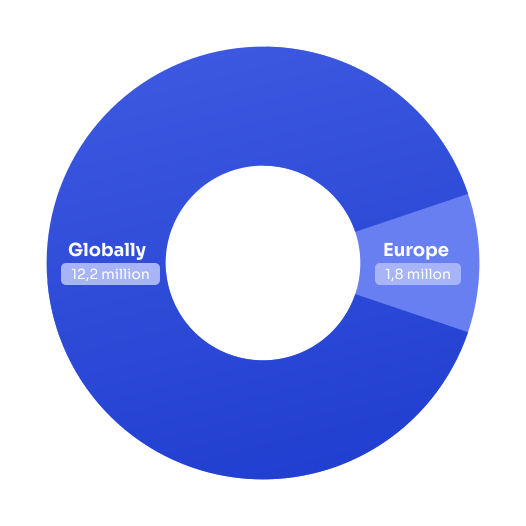
Stroke represents one of the world’s most significant health challenges: over 12.2 million new strokes occur globally each year, with 1.8 million new cases annually in Europe.
Stroke is the second leading cause of death worldwide and the third leading cause of disability. In Europe, 460,000 people die from stroke annually, while 9.5 million stroke survivors live on the continent.
The Critical Importance of Time
Time is crucial in stroke treatment: for every minute of delay, 2 million brain cells die.
Current stroke recognition and treatment statistics reveal:
- Emergency medical services correctly identify stroke in only 57-80% of cases
- Only 28% of patients reach hospital within the first “golden hour”
- Less than 5% receive treatment within the first 60 minutes
Research clearly demonstrates that treatment within 90 minutes dramatically improves outcomes: early treatment provides 40% greater chance of good functional outcome.
The 19% 3-year risk for patients treated within 90 minutes compared to 23.3% risk for delayed treatment.
57-80%
Correct identification by emergency services
28%
Reach hospital within golden hour
57-80%
Receie treatment within 60 minutes

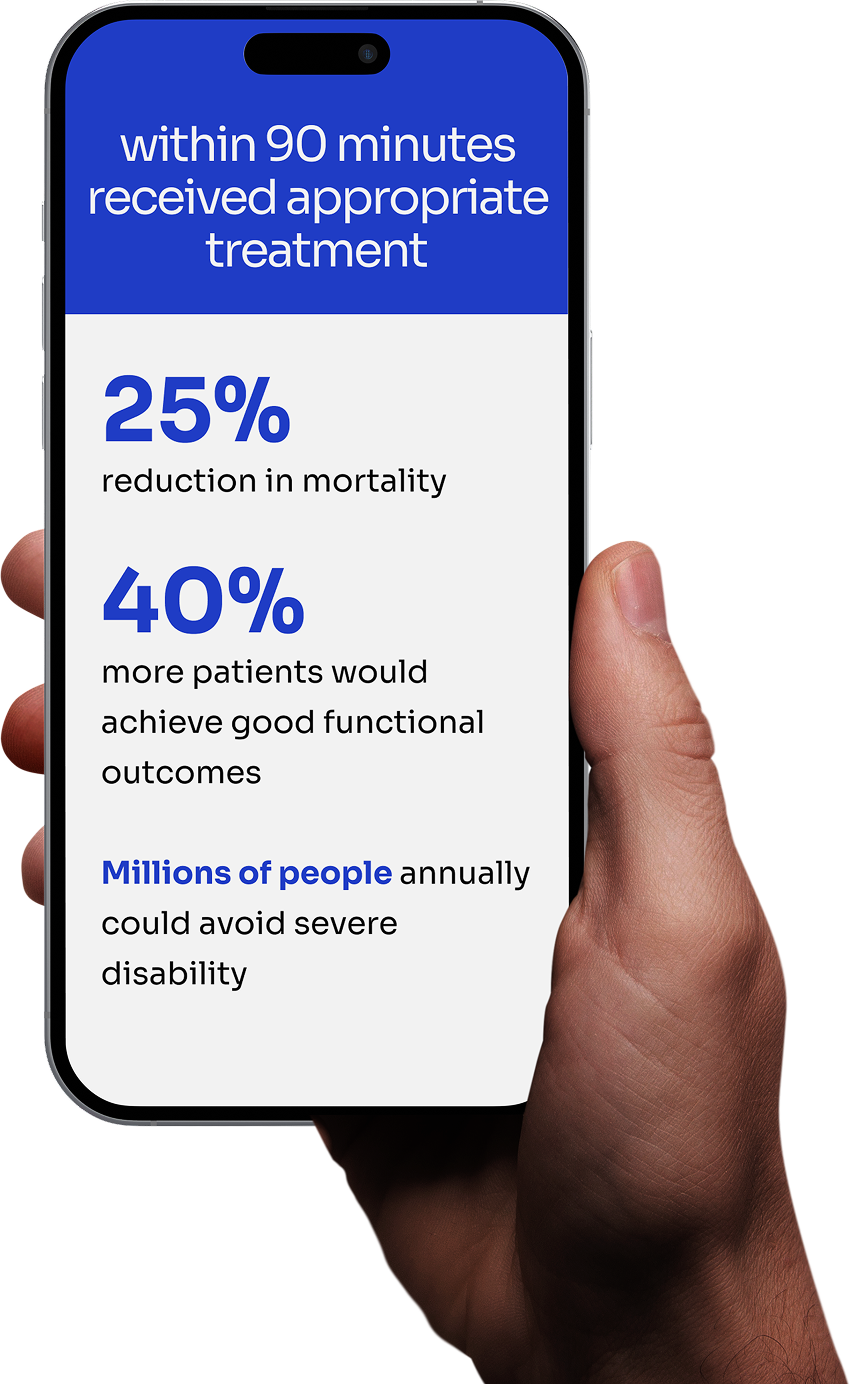
The aim of our research is to create a medical device that can identify and signal stroke-specific motor disturbances within 90 minutes.
This means the possibility of life—or independent, disability-free living after stroke—for millions of people worldwide! Thank you for supporting us!
The SalusmoPro Research
The clinical research includes healthy individuals, those at risk of stroke, and stroke survivors to ensure robust algorithmic performance. The study includes detailed medical consultation conducted by qualified medical researchers. Participation in the study is voluntary and completely free of charge.
SalusmoPro is being developed in full compliance with European MDR (Medical Device Regulation) and US FDA requirements, supporting future certification and market entry. All phases – from data collection and user participation to algorithm development – follow strict scientific, ethical, and data protection (GDPR) regulations, including official clinical trial permissions and anonymized data processing.
The essence of the project: volunteers contribute motion data in a home or clinical setting using wearable devices. These data are analyzed by AI to identify early signs of stroke, improving sensitivity and specificity. The collected data inform regulatory submissions and support the device’s safety and effectiveness profile as required by medical device authorities. All procedures and documentation align with the requirements of the National Public Health and Pharmaceutical Center (NNGYK) and international standards.
SalusmoPro is more than a technology project: it’s a science-driven initiative to shape the future of stroke diagnostics, guided by legal, medical, and ethical standards.
